

Yes. By definition, a nucleophile is an electron donor, and technically, so is a Lewis base. Nucleophilic activity is said to be for the purpose of bonding, whereas basic activity is said to be for the purpose of gaining a proton. However, a single nucleophile may act either way, depending on the context, and organic chemists bother to distinguish between these two activities. :)
A nucleophile donates electrons, tending to typically initiate a reaction. Maybe it is said that it "backside attacks" a ketone, or maybe it donates electrons to the antibonding orbital of bromine (first step of bromination of an alkene). Or, maybe it donates electrons to grab a proton off the alpha carbon of a ketone. Either way, it donates electrons.
A Lewis base is an electron donor as well, and it is typically taught in the context of Bronsted acids/bases, which are proton donors and acceptors, respectively. In that context, Lewis bases donate electrons specifically to get protons. However, that is not the only thing they can do, but rather, a simple case of their activity.
In some sense, "nucleophile" is a contextual "replacement" term for the organic chemist, in lieu of "Lewis base".
In contrast, a nucleophile is defined in a context with an electrophile to emphasize the fact that in any reaction with a nucleophile, there is an electrophile---an electron lover. An electrophile is also similar to a Lewis acid, like one would expect; it wants more electrons and therefore may be the target of a nucleophilic attack.
Note, however, that at some point you will have to distinguish between basic activity and nucleophilic activity (whether a nucleophile donates electrons simply to get a proton, or whether a nucleophile donates electrons in order to actually bond with another atom), so keep that in mind.
Here, a nucleophile acts as a base:
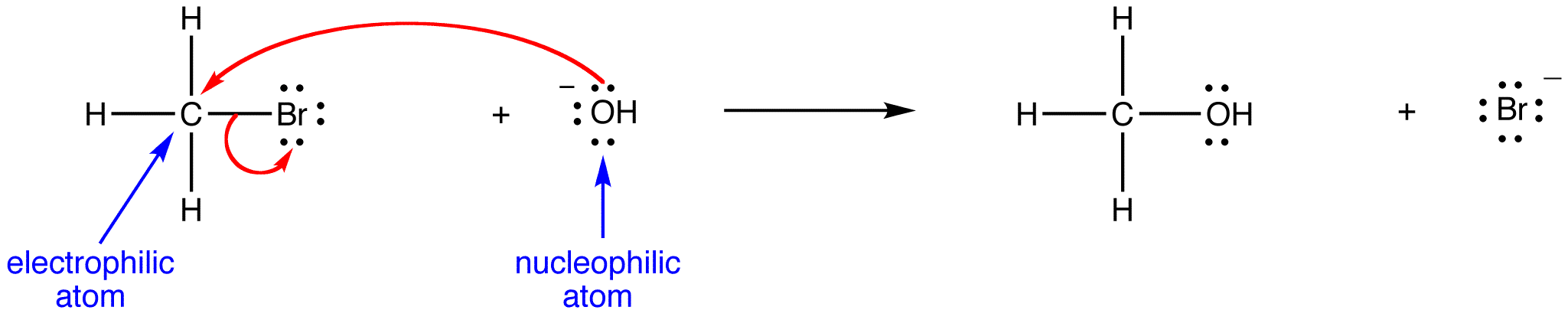
Here, the same nucleophile acts as a nucleophile: